Novel Method for Electroless Etching of 6H–SiC
Abstract
:1. Introduction
2. Materials and Methods
3. Results
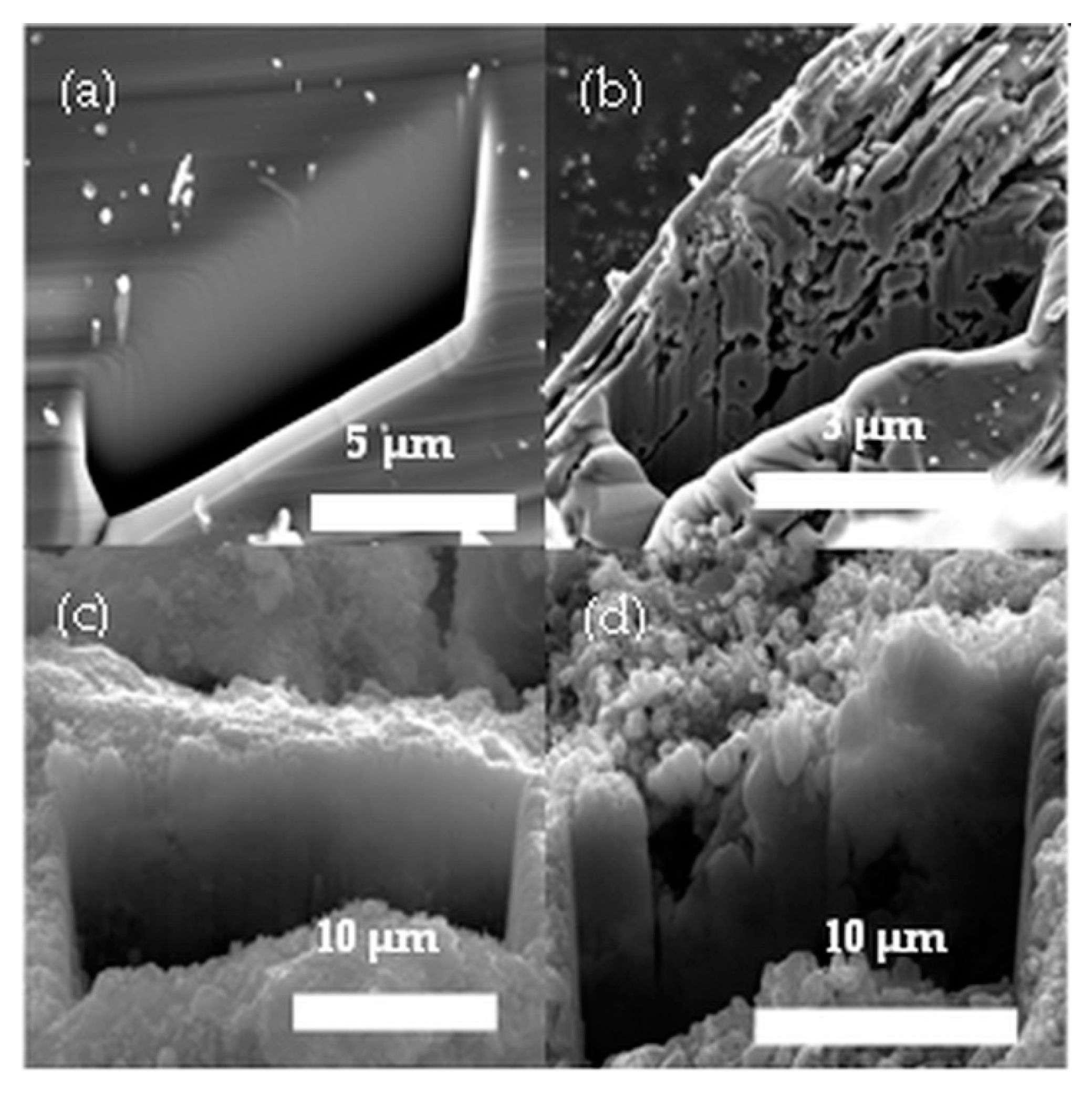
3.1. Chemical Etching and Morphology of the Bulk 6H-SiC
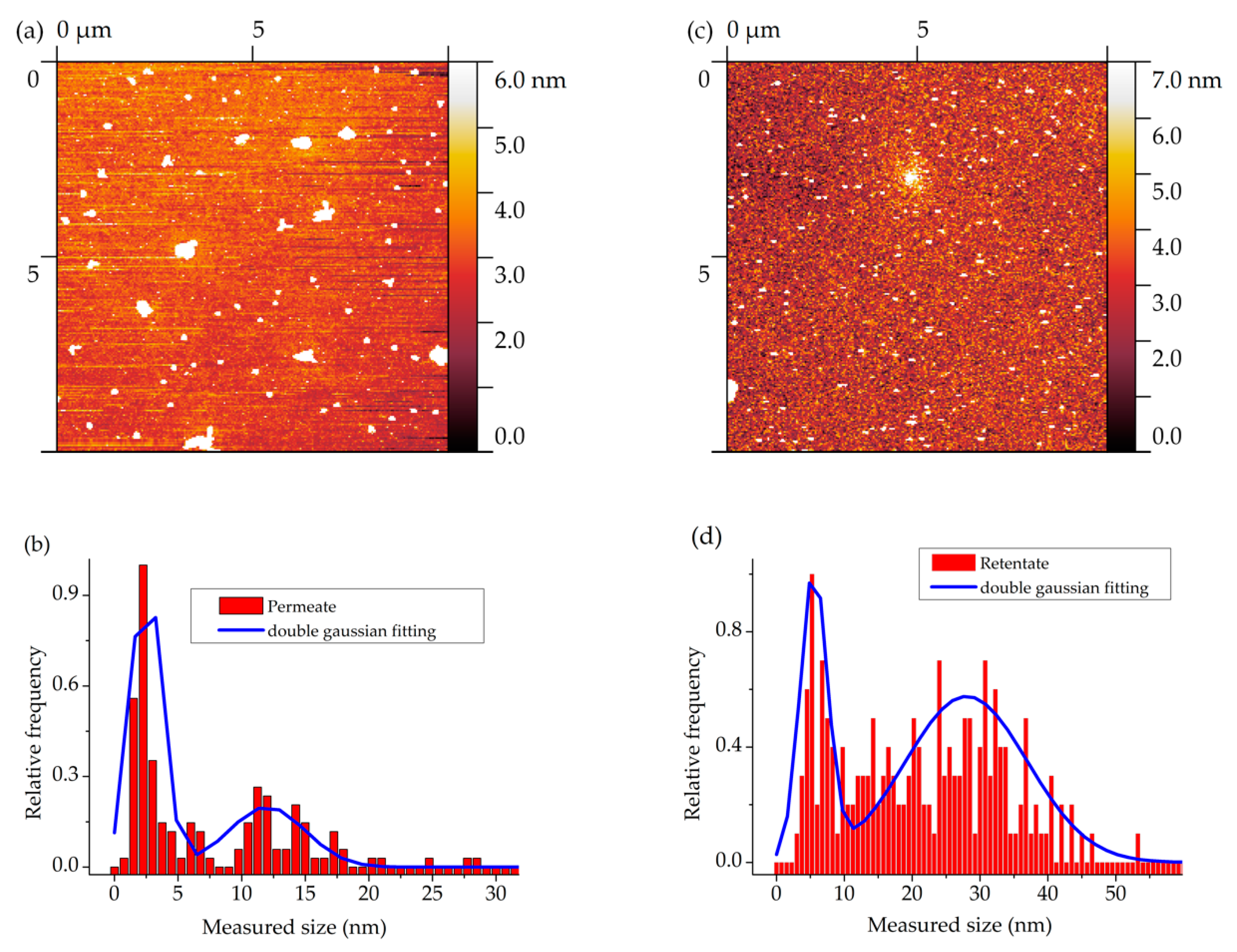
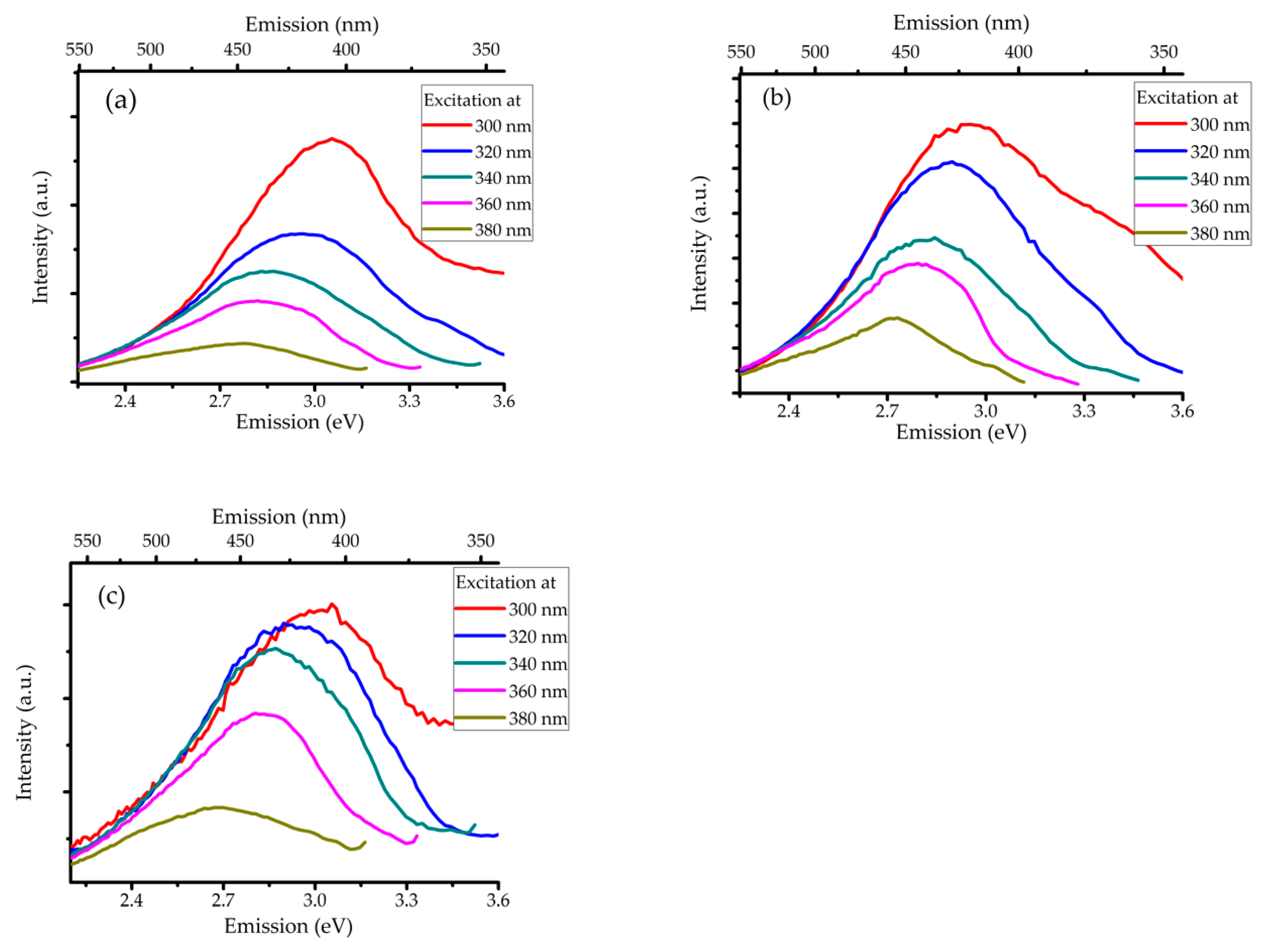
3.2. Characterizing the 6H Nanoparticles
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wellmann, P.; Svensson, B.G.; Sun, J.; Liu, X.; Løvvik, O.M.; Linnarsson, M.; Syväjärvi, M.; Johansen, B.A.; Jokubavicius, V.; Diplas, S.; et al. Cubic silicon carbide as a potential photovoltaic material. Sol. Energy Mater. Sol. Cells 2015, 145, 104–108. [Google Scholar] [CrossRef] [Green Version]
- Oku, T.; Matsumoto, T.; Hiramatsu, K.; Yasuda, M. Construction and characterization of spherical si solar cells combined with SiC electric power inverter. AIP Conf. Proc. 2017, 79, 79–83. [Google Scholar] [CrossRef] [Green Version]
- Gali, Á. Time-dependent density functional study on the excitation spectrum of point defects in semiconductors. Phys. Stat. Sol. B 2011, 1346, 1337–1346. [Google Scholar] [CrossRef]
- Widmann, M.; Lee, S.; Rendler, T.; Son, N.T.; Fedder, H.; Paik, S.; Yang, L.; Zhao, N.; Yang, S.; Booker, I.; et al. Coherent control of single spins in silicon carbide at room temperature. Nat. Mater. 2015, 14, 164–168. [Google Scholar] [CrossRef] [Green Version]
- Koehl, W.F.; Buckley, B.B.; Heremans, F.J.; Calusine, G.; Awschalom, D.D. Room temperature coherent control of defect spin qubits in silicon carbide. Nature 2011, 479, 84–87. [Google Scholar] [CrossRef]
- Weber, J.R.; Koehl, W.F.; Varley, J.B.; Janotti, A.; Buckley, B.B.; van de Walle, C.G.; Awschalom, D.D. Quantum computing with defects. Proc. Natl. Acad. Sci. USA 2010, 107, 8513–8518. [Google Scholar] [CrossRef] [Green Version]
- Castelletto, S.; Johnson, B.C.; Ivády, V.; Stavrias, N.; Umeda, T.; Gali, A.; Ohshima, T. A silicon carbide room-temperature single-photon source. Nat. Mater. 2013, 13, 151–156. [Google Scholar] [CrossRef] [Green Version]
- Castelletto, S.; Johnson, B.; Zachreson, C.; Beke, D.; Balogh, I.; Ohshima, T.; Aharonovich, I.; Gali, Á. Room temperature quantum emission from cubic silicon carbide nanoparticles. ACS Nano 2014, 8, 7938–7947. [Google Scholar] [CrossRef]
- Astakhov, G.V.; Simin, D.; Dyakonov, V.; Yavkin, B.V.; Orlinskii, S.B.; Proskuryakov, I.I.; Anisimov, A.N.; Soltamov, V.A.; Baranov, P.G. Spin centres in SiC for quantum technologies. Appl. Magn. Reson. 2016, 47, 793–812. [Google Scholar] [CrossRef]
- Beke, D.; Szekrényes, Z.; Pálfi, D.; Róna, G.; Balogh, I.; Maák, P.A.; Katona, G.; Czigány, Z.; Kamarás, K.; Rózsa, B.; et al. Silicon carbide quantum dots for bioimaging. J. Mater. Res. 2013, 28, 205–209. [Google Scholar] [CrossRef] [Green Version]
- Fan, J.; Li, H.; Jiang, J.; So, L.K.Y.; Lam, Y.W.; Chu, P.K. 3C–SiC nanocrystals as fluorescent biological labels. Small 2008, 4, 1058–1062. [Google Scholar] [CrossRef]
- Cao, T.; Cheng, Y.; Zhang, H.; Yan, B.; Cheng, Y. High rate fabrication of room temperature red photoluminescent SiC nanocrystals. Mater. Chem. C 2015, 3, 4876–4882. [Google Scholar] [CrossRef]
- Serdiuk, T.; Lysenko, V.; Skryshevsky, V.A.; Géloën, A. Vapor phase mediated cellular uptake of sub 5 nm nanoparticles. Nanoscale Res. Lett. 2012, 7, 212. [Google Scholar] [CrossRef] [Green Version]
- Botsoa, J.; Lysenko, V.; Geloen, A.; Marty, O.; Bluet, J.; Guillot, G.; Botsoa, J.; Lysenko, V.; Geloen, A.; Marty, O.; et al. Application of 3C–SiC quantum dots for living cell imaging. Appl. Phys. Lett. 2008, 92, 137902. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, Z.; Wu, X.L.; Xu, L.L.; Zhang, W.C.; Chu, P.K. Luminescent small-diameter 3C–SiC nanocrystals fabricated via a simple chemical etching method. Nanotechnology 2007, 18, 365603. [Google Scholar] [CrossRef]
- Cambaz, G.Z.; Yushin, G.N.; Gogotsi, Y.; Lutsenko, V.G. Anisotropic etching of SiC whiskers. Nano Lett. 2006, 6, 548–551. [Google Scholar] [CrossRef]
- Cao, A.T.; Luong, Q.N.T.; Dao, C.T. Influence of the anodic etching current density on the morphology of the porous SiC layer. AIP Adv. 2014, 4, 037105. [Google Scholar] [CrossRef]
- Leitgeb, M.; Zellner, C.; Schneider, M.; Schwab, S.; Hutter, H.; Schmid, U. Metal assisted photochemical etching of 4H silicon carbide. J. Phys. D 2017, 50, 435301. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, C.; Li, L.; Zhou, S.; Chen, X.; Gao, J.; Zhao, N.; Wong, C. Hybrid anodic and metal-assisted chemical etching method enabling fabrication of silicon carbide nanowires. Small 2019, 15, 1803898. [Google Scholar] [CrossRef]
- Syväjärvi, M.; Yakimova, R.; Hylén, A.L.; Janzén, E. Anisotropy of dissolution and defect revealing on SiC surfaces. J. Phys. Condens. Matter 1999, 11, 10041–10046. [Google Scholar] [CrossRef]
- Siche, D.; Klimm, D.; Hölzel, T.; Wohlfart, A. Reproducible defect etching of SiC single crystals. J. Cryst. Growth 2004, 270, 1–6. [Google Scholar] [CrossRef]
- Weyher, J.L.; Lazar, S.; Borysiuk, J.; Pernot, J. Defect-selective etching of SiC. Phys. Status Solidi Appl. Mater. Sci. 2005, 202, 578–583. [Google Scholar] [CrossRef]
- Konstantinov, A.O.; Harris, C.I.; Janzén, E. Electrical properties and formation mechanism of porous silicon carbide. Appl. Phys. Lett. 1994, 65, 2699–2701. [Google Scholar] [CrossRef]
- Shishkin, Y.; Choyke, W.J.; Devaty, R.P. Photoelectrochemical etching of n-type 4H silicon carbide. J. Appl. Phys. 2004, 96, 2311–2322. [Google Scholar] [CrossRef]
- Shishkin, Y.; Ke, Y.; Devaty, R.P.; Choyke, W.J. Fabrication and morphology of porous p-type SiC. J. Appl. Phys. 2005, 97, 044908. [Google Scholar] [CrossRef]
- Beke, D.; Károlyházy, G.Y.; Czigány, Z.; Bortel, G.; Kamarás, K.; Gali, Á. Harnessing no-photon exciton generation chemistry to engineer semiconductor nanostructures. Sci. Rep. 2017, 7, 10599. [Google Scholar] [CrossRef] [Green Version]
- Alekseev, S.; Korytko, D.; Iazykov, M.; Khainakov, S.; Lysenko, V. Electrochemical Synthesis of Carbon Fluorooxide Nanoparticles from 3C–SiC Substrates. J. Phys. Chem. C 2015, 119, 20503–20514. [Google Scholar] [CrossRef]
- Contarini, S.; Howlett, S.P.; Rizzo, C.; Angelis, B.A.D. XPS study on the dispersion of carbon additives in silicon carbide powders. Appl. Surf. Sci. 1991, 51, 177–183. [Google Scholar] [CrossRef]
- Szekrényes, Z.; Somogyi, B.; Beke, D.; Károlyházy, G.Y.; Balogh, I.; Kamarás, K.; Gali, Á. Chemical transformation of carboxyl groups on the surface of silicon carbide quantum dots. J. Phys. Chem. C 2014, 118, 19995–20001. [Google Scholar] [CrossRef] [Green Version]
- Delplancke, M.P.; Powers, J.M.; Vandentop, G.J.; Salmeron, M.; Somorjai, G.A.; Delplancke, M.P.; Powers, J.M.; Vandentop, G.J.; Salmeron, M.; Somorjai, G.A. Preparation and characterization of amorphous SiC: H thin films. J. Vac. Sci. Technol. A 2002, 9, 450–455. [Google Scholar] [CrossRef] [Green Version]
- Waldrop, J.R.; Grant, R.W.; Wang, Y.C.; Metal, R.F.D. Schottky barrier contacts to alpha 6H-SiC. J. Appl. Phys. 1992, 72, 4757–4760. [Google Scholar] [CrossRef]
- Rossi, A.M.; Murphy, T.E.; Reipa, V. Ultraviolet photoluminescence from 6H silicon carbide nanoparticles. Appl. Phys. Lett. 2008, 92, 253112. [Google Scholar] [CrossRef] [Green Version]
- Beke, D.; Szekrényes, Z.; Czigány, Z.; Kamarás, K.; Gali, Á. Dominant luminescence is not due to quantum confinement in molecular-sized silicon carbide nanocrystals. Nanoscale 2015, 7, 10982–10988. [Google Scholar] [CrossRef] [Green Version]

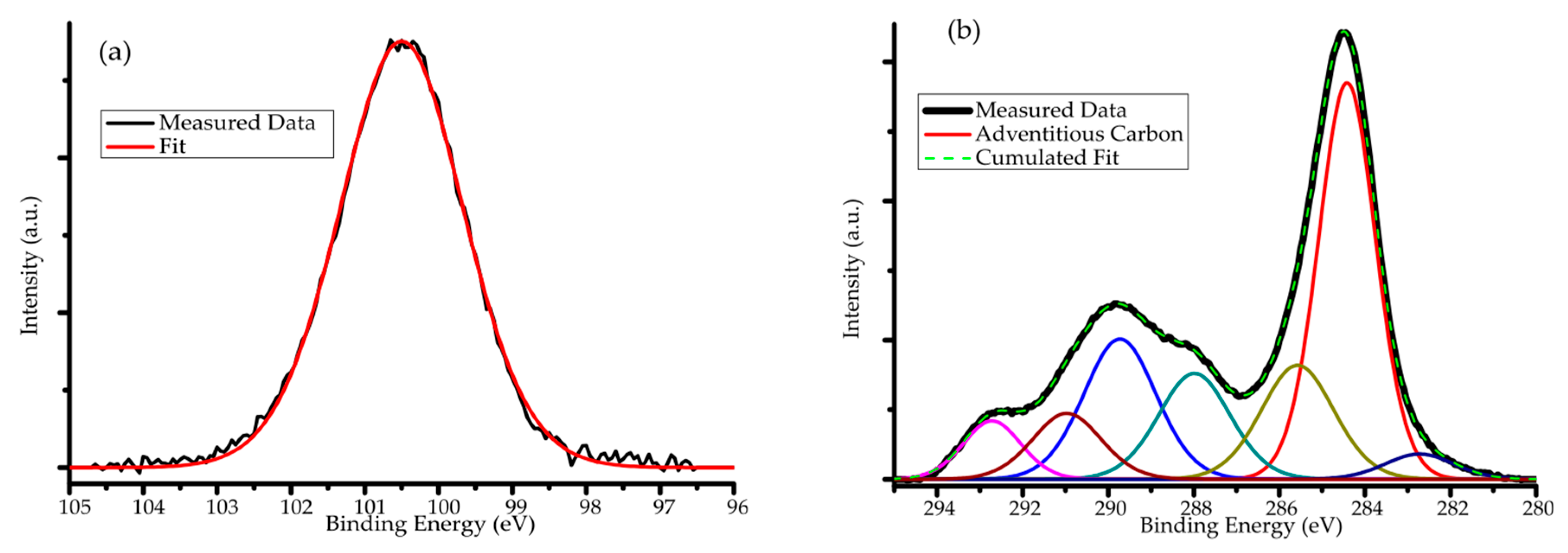
| Binding Energy | Chemical Bond or Group |
|---|---|
| 284.5 eV | Adventitious carbon |
| 289.8 eV | C=O group, C=S group |
| 288.1 eV | C=O group |
| 292.8 eV | C–F group |
| 285.7 eV | C–C, C–H bond |
| 282.8 eV | Si–C bond (shifted by 5.3 eV in total) |
| 291.1 eV | CO32− ion |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Károlyházy, G.; Beke, D.; Zalka, D.; Lenk, S.; Krafcsik, O.; Kamarás, K.; Gali, Á. Novel Method for Electroless Etching of 6H–SiC. Nanomaterials 2020, 10, 538. https://doi.org/10.3390/nano10030538
Károlyházy G, Beke D, Zalka D, Lenk S, Krafcsik O, Kamarás K, Gali Á. Novel Method for Electroless Etching of 6H–SiC. Nanomaterials. 2020; 10(3):538. https://doi.org/10.3390/nano10030538
Chicago/Turabian StyleKárolyházy, Gyula, Dávid Beke, Dóra Zalka, Sándor Lenk, Olga Krafcsik, Katalin Kamarás, and Ádám Gali. 2020. "Novel Method for Electroless Etching of 6H–SiC" Nanomaterials 10, no. 3: 538. https://doi.org/10.3390/nano10030538
APA StyleKárolyházy, G., Beke, D., Zalka, D., Lenk, S., Krafcsik, O., Kamarás, K., & Gali, Á. (2020). Novel Method for Electroless Etching of 6H–SiC. Nanomaterials, 10(3), 538. https://doi.org/10.3390/nano10030538





